A nanocarrier system to effectively deliver anti-inflammatory drugs and improve the treatment of inflammatory diseases
Background
The inflammatory response is the body’s natural defense mechanism against infection and injury. In certain instances, however, persistent and uncontrolled inflammation becomes detrimental and contributes to the development and progression of various debilitating diseases, including cancer, atherosclerosis, autoimmune and neurodegenerative disorders. Anti-inflammatory drugs are considered a viable strategy for preventing and treating inflammation. However, many small-molecule anti-inflammatory drugs suffer from limited aqueous solubility in physiological fluids, in vivo instability, and non-specific biodistribution, resulting in relatively low bioavailability, off-target effects, and limited therapeutic efficacy. Although currently available nanoparticle-based drug delivery systems can address some of these limitations, their efficient systemic distribution to inflamed tissue still remains a challenge. To mitigate this issue, some nanoparticles have been designed to deliver anti-inflammatory drugs via local routes, including oral administration for inflammatory bowel disease, intra-articular injection for arthritis, and intranasal administration for lung inflammation. However, these delivery strategies are limited to the treatment of accessible inflammation sites. Therefore, nanocarriers that efficiently accumulate in inflamed tissues following systemic administration are essential to develop highly efficacious formulations for anti-inflammatory drugs.
Technology Description
A VCAM-1 targeted nanoparticle-based platform for systemic delivery of inflammation inhibitors to inflamed tissues that dramatically increase the intrinsic aqueous solubility of anti-inflammatory compounds. In one example, a clinically tested IRAK4 inhibitor, PF-06650833, that otherwise has a limited aqueous solubility (57 µg mL-1), is successfully delivered to inflamed tissues. The developed IRAK4 nanocarriers increase the intrinsic aqueous solubility of this PF-06650833 by 40 times. A vascular cell adhesion molecule 1 (VCAM1) targeting peptide on the surface of nanocarriers significantly enhances their accumulation after intravenous injection in inflamed tissues of mice with induced paw edema and ulcerative colitis when compared to non-targeted counterparts. The nanocarrier delivered IRAK4 inhibitor markedly abates inflammation and dramatically suppresses paw edema, mitigates colitis symptoms, and reduces proinflammatory cytokine levels in the affected tissues. Importantly, repeated intravenous injections of IRAK4 inhibitor-loaded nanocarriers have no acute toxic effect on major organ systems of mice. Therefore, the developed targeted nanocarriers have the potential to significantly improve the therapeutic efficacy of IRAK4 inhibitors for the treatment of various inflammatory diseases.
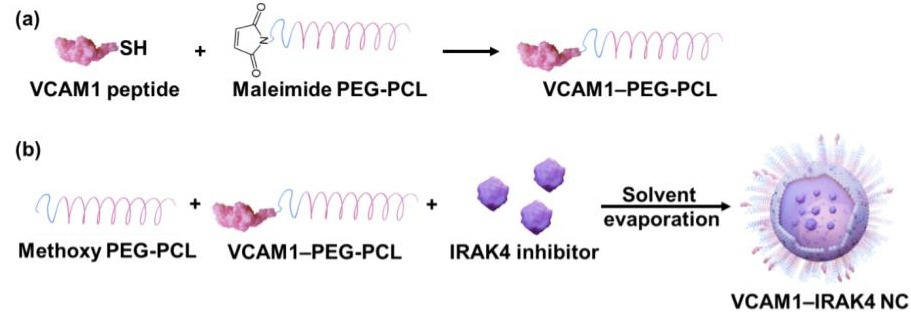
Figure 1. The two-step preparation procedure for VCAM1-targeted nanocarriers loaded with the IRAK4 inhibitor (VCAM1-IRAK4 NC). (a) Step 1: Conjugation of the VCAM1 peptide to maleimide-terminated PEG-PCL via a thioether bond. (b) Step 2: Encapsulation of the IRAK4 inhibitor in polymeric nanocarriers composed of the synthesized VCAM1-PEG-PCL and methoxy-PEG-PCL. (PEG ‑ polyethylene glycol, PCL ‑ polycaprolactone)
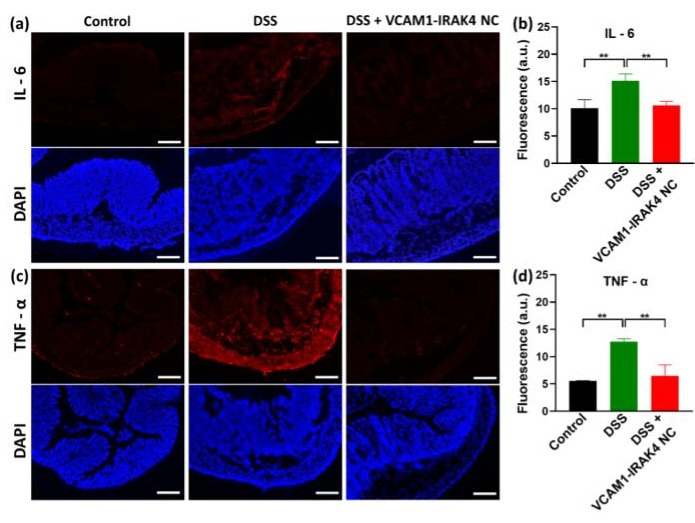
Figure 2. Representative fluorescence microscope images of sectioned colon tissues. (a and c) Representative fluorescence microscope images of sectioned colon tissues that have been stained for IL-6 (a) and TNF-α (c). Scale bars = 200 μm. Examined colons were collected from healthy mice (Control), and DSS-induced colitis mice treated with saline (DSS) and VCAM1-IRAK4 NC (DSS + VCAM1-IRAK4 NC). Scale bars = 200 µm. (b and d) Mean fluorescence signals from the mouse colons stained for IL-6 (b) and TNF-α (d), respectively. Data shown are means ± standard deviation. Statistical comparisons between groups were performed with one-way ANOVA (n = 4, ** _p_ < 0.01, *** _p_ < 0.001).
Further Details: https://pubmed.ncbi.nlm.nih.gov/37702136/
Features & Benefits
- Increases intrinsic solubility and bio-availability of anti-inflammatory drugs
- Provides efficient accumulation in inflamed tissues
- Data suggests repeated injections do not induce toxicity
Applications
- Colitis
- Atherosclerosis
- Autoimmune disease
- Neurodegenerative disorders
- Cancer
- Cachexia
Opportunity
Oregon State University is seeking a licensee or development partner for non-exclusive or exclusive licensing.
Status
Provisional patent application filed